-40%
50 Pack - Fentanyl Instant Urine Drug Test Kit - 200 ng/mL
$ 26.39
- Description
- Size Guide
Description
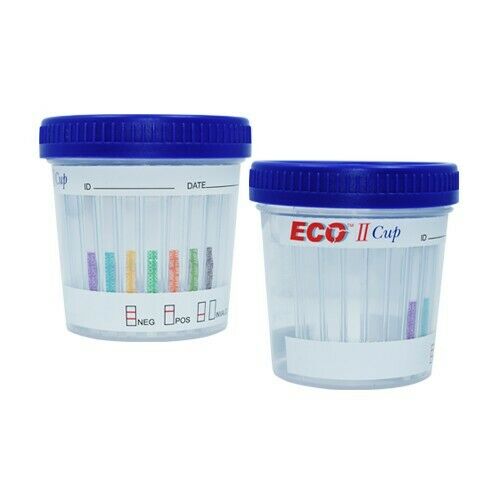
50 individually wrapped testsUrine Drug Test for Fentanyl Detection
Product Description:
An affordable workplace test to determine if employees are using opiods products
Easy-to-use test kit generating accurate results in minutes
Instructions
:
Have donor provide urine sample and let stand until it's room temperature. Open the foil pouch and remove the test device.
Remove the cap from the test device
Immerse the absorbent tip into the urine sample for 10-15 seconds. Urine sample should not touch the plastic device
Replace the cap over the absorbent tip and lay the device flatly on a non-absorptive clean surface
Read results in 5 minutes (do not interpret results after 10 minutes
Drug cut-off
:
FEN: Fentanyl - cut-off 200 ng/mL
Results
:
Positive:
One colored line appears in the control (C) region & no line appears in the test region (T).
Negative:
One colored line appears in the control (C) region, and another colored line in the test (T) region. The color intensity of the test line may be weaker or stronger than that of the control line, but regardless of the intensity, the result is negative.
Invalid:
No line appears in the control zone “C”. If the test device does not produce a band in the control region, check the testing procedures, samples, and/or control materials and repeat the test using a new device.